Synergy Pharmaceuticals Reports First Quarter 2013 Financial Results
Recent Developments
·On January 2, 2013, Synergy announced positive results from its large multicenter clinical trial of its lead investigational drug plecanatide in patients with CIC.
·On January 17, 2013, Synergy completed its merger with Callisto Pharmaceuticals, Inc.
·On March 15, 2013, Synergy announced that the full study results from its large multicenter CIC clinical trial would be featured in a late-breaking oral presentation session at Digestive Disease Week 2013 in Orlando, Florida on Tuesday, May 21, 2013.
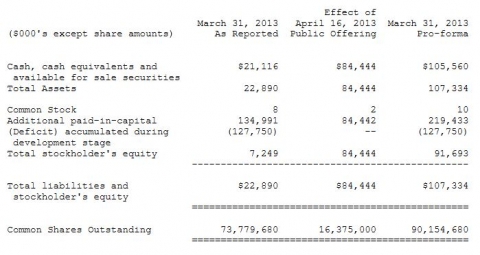
·On April 16, 2013, Synergy closed a public offering of 16,375,000 shares of its common stock at a price of $5.50 per share, less underwriting discounts and commissions. The net proceeds to Synergy from this sale was approximately $84.4 million, after deducting underwriting discounts and commissions and other estimated offering expenses payable by Synergy. (Pro-forma Balance Sheets below)
Financial Update
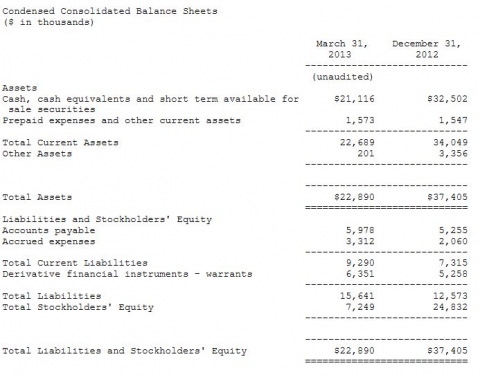
Synergy's cash, cash equivalents and short term available for sale securities balance as of March 31, 2013 was $21.1 million, as compared to $32.5 million on December 31, 2012. During the three months ended March 31, 2013 net cash provided by financing activities was $4.6 million from the controlled equity sales of its common stock, whereas there was no such financing activity during the three months ended March 31, 2012. Net cash used in operating activities during the three months ended March 31, 2013 and 2012 was $15.6 million and $6.8 million, respectively.
Net loss for the three months ended March 31, 2013 was $18.7 million or $0.26 per share, as compared to a net loss of $7.0 million, or $0.13 per share, for the three months ended March 31, 2012. During the three months ended March 31, 2012 non-cash expense items, principally the change in fair value of derivative instruments and share based compensation expense, totaled $2.4 million, or $0.03 per share, whereas such items in the three months ended March 31, 2012 totaled $.4 million, or $0.01 per share.
Synergy had approximately 73.8 million common shares outstanding at March 31, 2013.
About Synergy Pharmaceuticals Inc.
Synergy is a biopharmaceutical company focused on the development of new drugs to treat gastrointestinal disorders and diseases. Synergy‘s lead proprietary drug candidate plecanatide is a synthetic analog of the human gastrointestinal (GI) hormone uroguanylin, and functions by activating the guanylate cyclase C receptor on epithelial cells of the GI tract. Synergy previously completed a Phase I study of plecanatide in healthy volunteers and a Phase IIa clinical trial in CIC patients. On January 2, 2013, Synergy announced positive results in a Phase II/III large multicenter clinical trial of plecanatide to treat CIC. Plecanatide is also being developed to treat patients with IBS-C. Synergy’s second GC-C agonist SP-333 is in clinical development to treat inflammatory bowel diseases, and is presently in a Phase I trial in healthy volunteers. More information is available at http://www.synergypharma.com.
Website: http://www.synergypharma.com
Contact
Investor Contact Information
Danielle Spangler
The Trout Group
Send Email
(646) 378-2924
This news is a press release provided by Synergy Pharmaceuticals.
-
2013년 5월 21일 23:00
-
2013년 5월 15일 09:52