Dimedi, X-Ray Scanner ‘DMD D-2000’ acquires Venture Business and CE certification
- Korea’s first X-ray scanner development receives recognition for outstanding technical skills
- Europe·Canada·Australia Prepare bridgehead to advance into the Global Market
In accordance with article 25 of Special Procedures Regulations, Small and Medium Business Corporation evaluates and selects businesses that have outstanding technical skills or innovative capacity to nominate for venture business certification, which promotes venture businesses. DMD D-2000 Koreawide, is an ideally developed X-ray scanner that has received acknowledgement for its technical skills.
CE certification is Europe’s Harmonization of Standards certification mark. In order to export products to the European region, CE is an important mark. This acquired mark gives consumers security and health, hygiene, and protection of environment. Dimedi’s product meets the requirements of Harmonization of Standards.
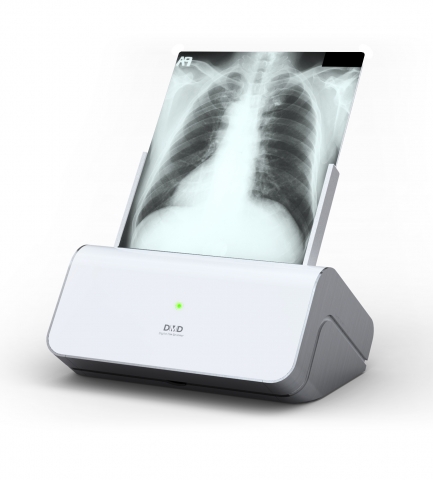
Acquisition of Venture business certification and CE certification for Dimedi’s DMD D-2000 is an ideal product for digitization of x-ray digitization in Korea. Existing cumbersome to store X-ray film, cast away from traditional transport method, massive material can be digitized and conveniently saved through PACS viewer on a PC which can be viewed and examined right away.
The approximate weight is 11kg and can be conveniently stored and used in small spaces. From basic film brightness adjustment, to auto sizing option which automatically detects film size, hospitals/medical facilities can conveniently store and use Dimedi’s DMD D-2000.
Also, it is possible to measure Growth Plate and Bone Mineral Density. Body parts such as the heel of your foot, wrist, fingers, etc., can be examined by checking growth plates. Genetic composition of parents along with the growth of their children can be estimated by calculating base genetic composition. In the case of Bone Mineral Density, simply input gender and age for measurements. This one stop system allows you to utilize various options conveniently.
Dimedi’s CEO, Nam Yun said “After extensive research, technical skills and device power through development, our certification can be verified. Korea, Europe, Canada, Australia, South America, etc., rely on Europe CE certification mark. This is a bridgehead to advance into the global market.”
Dimedi product DMD D-2000 is used in over 100 hospitals/medical facilities all over Korea, and is exported globally to Turkey, Mexico, Colombia, and Chile amongst many other countries.” (Inquiries: sales@dimedi.co.kr)
Contact
DIMEDI
Send Email
This news is a press release provided by Dimedi.